
NEWSLETTER
ISSUE
Oct to Dec, 2021 Volume 16
BRAIN WAVE
Dry Heat Sterilization / De-pyrogenation
Sterilization is a cornerstone of the pharmaceutical manufacturing process. Several sterilization methods are employed in the industry based on the process requirement. The sterilization process is broadly classified into Cold and Hot Sterilization. Standard cold sterilization methods used in the process are chemical sterilization, ionizing radiation, and UV radiation, whereas the hot method is further classified into Moist heat sterilization and Dry sterilization. The moist heat methods use different media such as steam, super-heated water or steam-air mixture. In contrast, the Dry heat method uses hot air for sterilization.
The dry heat method is employed for sterilization and de-pyrogenation of the material used in pharmaceutical processing. This method is preferred for materials that may be damaged by moist heat or that are impenetrable to moist heat. Fundamentally, the Dry Heat Sterilization method destroys the microorganisms using hot air at temperatures ranging from 160°C to 180°C for exposure time up to 2 hrs. Similarly, de-pyrogenation is carried out for complete destruction and removal of pyrogens using hot air at higher temperatures up to 300°C and exposure time up to 2 hrs, depending on the requirement. Dry Heat Sterilization and de-pyrogenation are generally used for materials such as Glass Bottles, Vials, Ampoules, Containers (Stainless Steel & Aluminum), powders like sulphonamides and talcum powder.

The dry heat method is non-toxic, environmentally safe and causes no pollution or waste to the surrounding. Moreover, it is noncorrosive for metallic components, and the system is easy to install.
The monitoring of the sterilization process is done through b. atrophaeus spores instead of G. stearothermophilus spores for dry heat sterilization as the former is comparatively more resistant to dry heat. The primary lethal process is the oxidation of cell constituents. The dry heat destroys microorganisms by causing the denaturation of proteins.
There are two types of dry-heat sterilizers a). Static-air type and b). Forced-air type. The static-air type sterilizer is slower in heating and takes longer to achieve the sterilization temperature. In addition, the temperature distribution across the chamber is not uniform in comparison to the forced-air type sterilizer. The forced-air type sterilizer is equipped with a motor-driven blower that circulates heated air throughout the chamber at relatively high velocity, resulting in effective and rapid heat transfer.
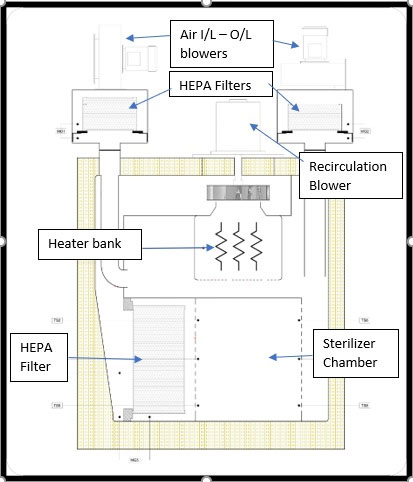
Construction:
The major components in a forced-air type sterilizer are air inlet and air outlet blowers, connected to the chamber via HEPA filters, a Heater bank for heating the air, HEPA filter for the chamber and the recirculation blower. The HEPA filter ensures the internal chamber environment by restricting the particle contamination from the recirculating air.
Process:
The process is carried out in several phases, starting from the drying phase, heat-up phase, stabilization phase, sterilization / de-pyrogenation phase, and cooling phase.
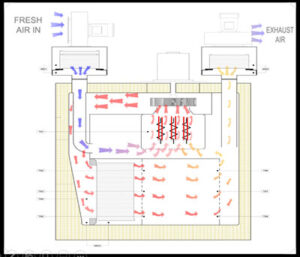

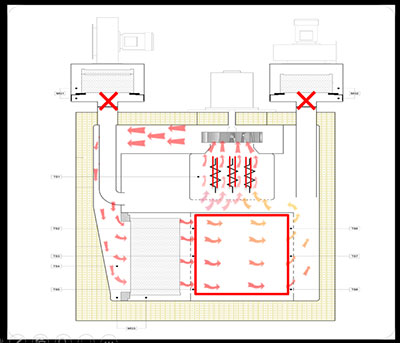
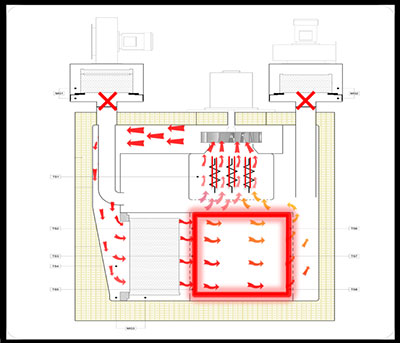

Drying Phase: The process begins with the drying phase, wherein the fresh air is circulated through the exhaust module and passed through the heater bank. During this phase, any traces of moisture are removed from the chamber.
Heat up & Stabilization Phase: The fresh air and exhaust modules are shut off after drying. The air within the chamber recirculates through the heater bank & chamber HEPA filter. The heated air gradually increases the chamber temperature. Upon attaining the preset temperature, the stabilization phase continues until the uniform temperature is achieved within the chamber.
Sterilization / De-pyrogentation Phase: Once the chamber temperature stabilizes at the preset temperature (up to 300°C), the sterilization hold time starts and continues until the preset time. In the case of sterilization, the microorganisms are destroyed, and in de-pyrogenation, complete destruction and removal of the pyrogen take place during this phase.
Cooling Phase: Upon completing the sterilization/de-pyrogenation phase, the fresh air & exhaust module is open to cool down the load and the chamber.
The modern dry heat sterilization / de-pyrogenation systems can operate the process in fully auto mode to ensure smooth and effective sterilization.

Reference
Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008
Subscribe to our Newsletter
Stay tuned with Industry updates
| Thank you for Signing Up |

Contact:
ARCHIVE
About
Who we are and what we do.
